Question 246Steam Plants - Assistant Engineer
70% to passAccording to the data given in illustration, which of the following would be the physical state of the fluid at a gage vacuum of 28.09 inches Hg, and 117.99 degrees Fahrenheit? Illustration SG-0026
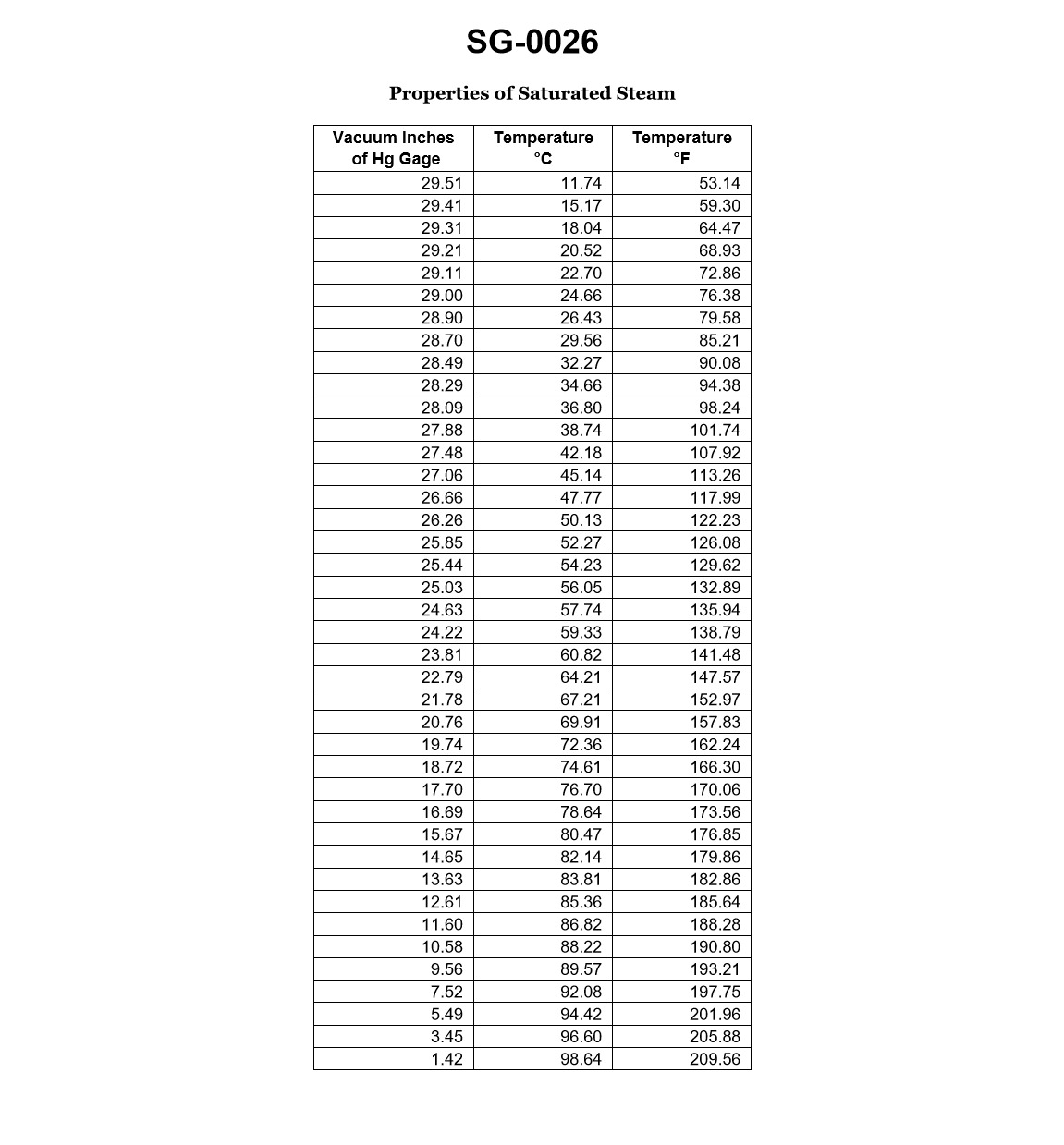
ASub cooled liquid.
BSaturated liquid.
CSuperheated vapor.
DMixture of saturated liquid and vapor.
AI Explanation
The correct answer is C) Superheated vapor. At a gage vacuum of 28.09 inches Hg and a temperature of 117.99 degrees Fahrenheit, the fluid would be in a superheated vapor state. This is because the given conditions place the fluid above the saturation line on a pressure-temperature diagram, indicating that the fluid is in a superheated state. The other options are incorrect because a gage vacuum of 28.09 inches Hg corresponds to a low pressure, which would not support a sub-cooled liquid (A) or a saturated liquid (B) state. Additionally, a temperature of 117.99 degrees Fahrenheit is too high for a mixture of saturated liquid and vapor (D) to exist at the given pressure.
Related Questions
Q66:Steam drum water level indicators must be calibrated to compensate for density d... Q380:Before an explosion can occur in a boiler furnace, there must be an accumulation... Q112:Condensate accumulation in the steam side of a fuel oil heater could result in _... Q197:A slight vacuum is maintained in the shell of the first stage heater shown in th... Q618:One function of the disks, in a disk-type centrifugal purifier, is to divide the...
Ready to test your knowledge?
Take a Steam Plants - Assistant Engineer Practice ExamOfficial Resources