Question 39 OSE01 - Chief Engineer - OSV
Due to environmental and safety concerns, the towing winch drive diesel engine cooling water system is treated with propylene glycol for protection against freezing. According to the illustration, what would be the limit of protection if 40 pints of propylene glycol are used in treating a cooling water system with a volumetric capacity of 10 gallons? Illustration MO-0209
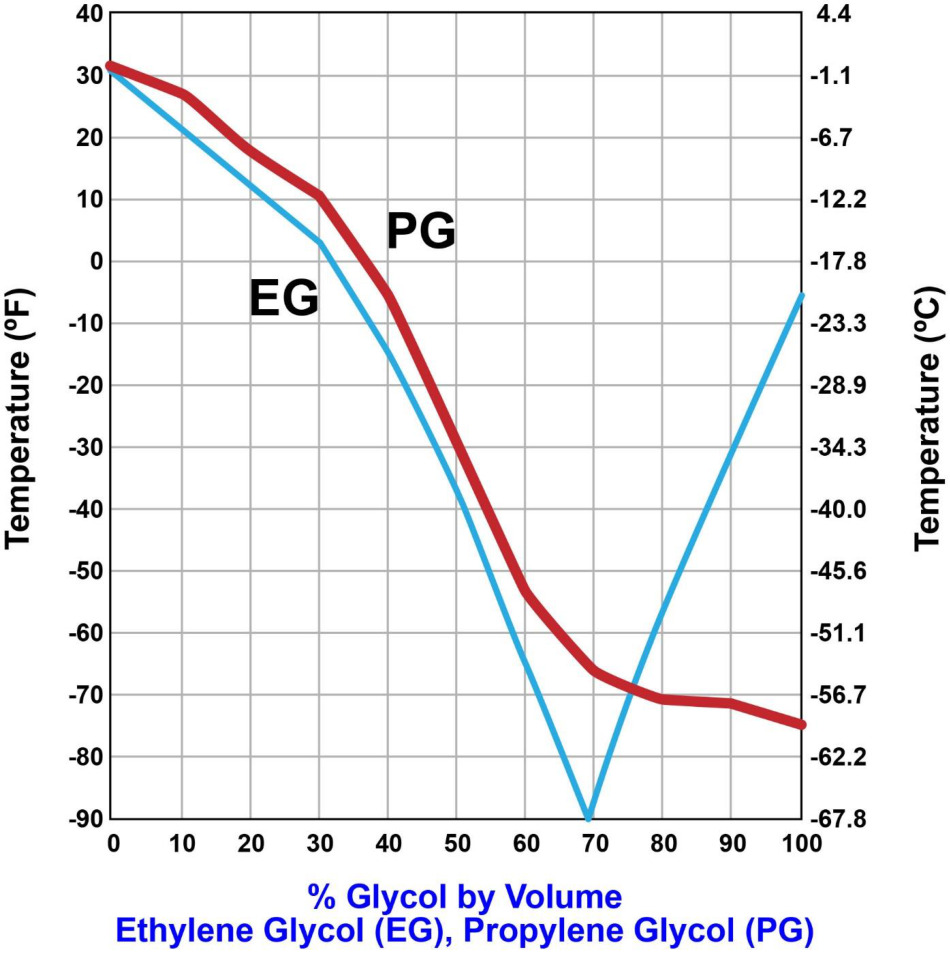
The Correct Answer is C ### Explanation for Option C (-30°F) The problem requires determining the freezing protection limit based on the concentration of propylene glycol (PG) in a cooling water system, referencing a specific, unprovided illustration (MO-0209) which typically contains a concentration-vs-freezing point chart. Since the chart is missing, we must rely on standard conversion factors and concentration percentages used in such scenarios. **Step 1: Convert all volumes to a consistent unit.** * **System Capacity:** 10 gallons. * **Propylene Glycol Used:** 40 pints. We convert pints to gallons: $$1 \text{ gallon} = 8 \text{ pints}$$ $$\text{PG Volume} = \frac{40 \text{ pints}}{8 \text{ pints/gallon}} = 5 \text{ gallons}$$ **Step 2: Calculate the concentration percentage of propylene glycol (PG).** The concentration (by volume) is the volume of PG divided by the total system capacity: $$\text{PG Concentration} = \frac{\text{PG Volume}}{\text{Total Capacity}} \times 100\%$$ $$\text{PG Concentration} = \frac{5 \text{ gallons}}{10 \text{ gallons}} \times 100\% = 0.50 \times 100\% = 50\%$$ **Step 3: Determine the freezing point based on the 50% concentration.** Standard engineering tables and charts (like the hypothetical Illustration MO-0209) for a 50\% volume concentration of propylene glycol (mixed with water) typically indicate a freezing protection limit of approximately **$-30^\circ\text{F}$** (or $-34^\circ\text{C}$). Therefore, the limit of protection is $-30^\circ\text{F}$. --- ### Explanation of Incorrect Options **A) 10°F:** A freezing point of $10^\circ\text{F}$ corresponds to a very low concentration of antifreeze (typically less than 20% PG). Since the concentration calculated is 50%, this level of protection is too low. **B) -6°F:** A freezing point of $-6^\circ\text{F}$ corresponds to a concentration of approximately 30-35% propylene glycol. Since 50% PG was added, the actual protection is significantly greater than this value. **D) -53°F:** A freezing point of $-53^\circ\text{F}$ corresponds to a concentration of approximately 60% propylene glycol. Since the concentration calculated is exactly 50%, this level of protection is unattainable with the volume of PG used. (Note: $60\%$ concentration often represents the lowest practical freezing point achievable with PG, known as the eutectic point for some blends, but it requires more PG than was added.)
Pass Your Coast Guard Licensing Exams!
Study offline, track your progress, and simulate real exams with the Coast Guard Exams app