Question 231Steam Plants - 1st Asst/Chief
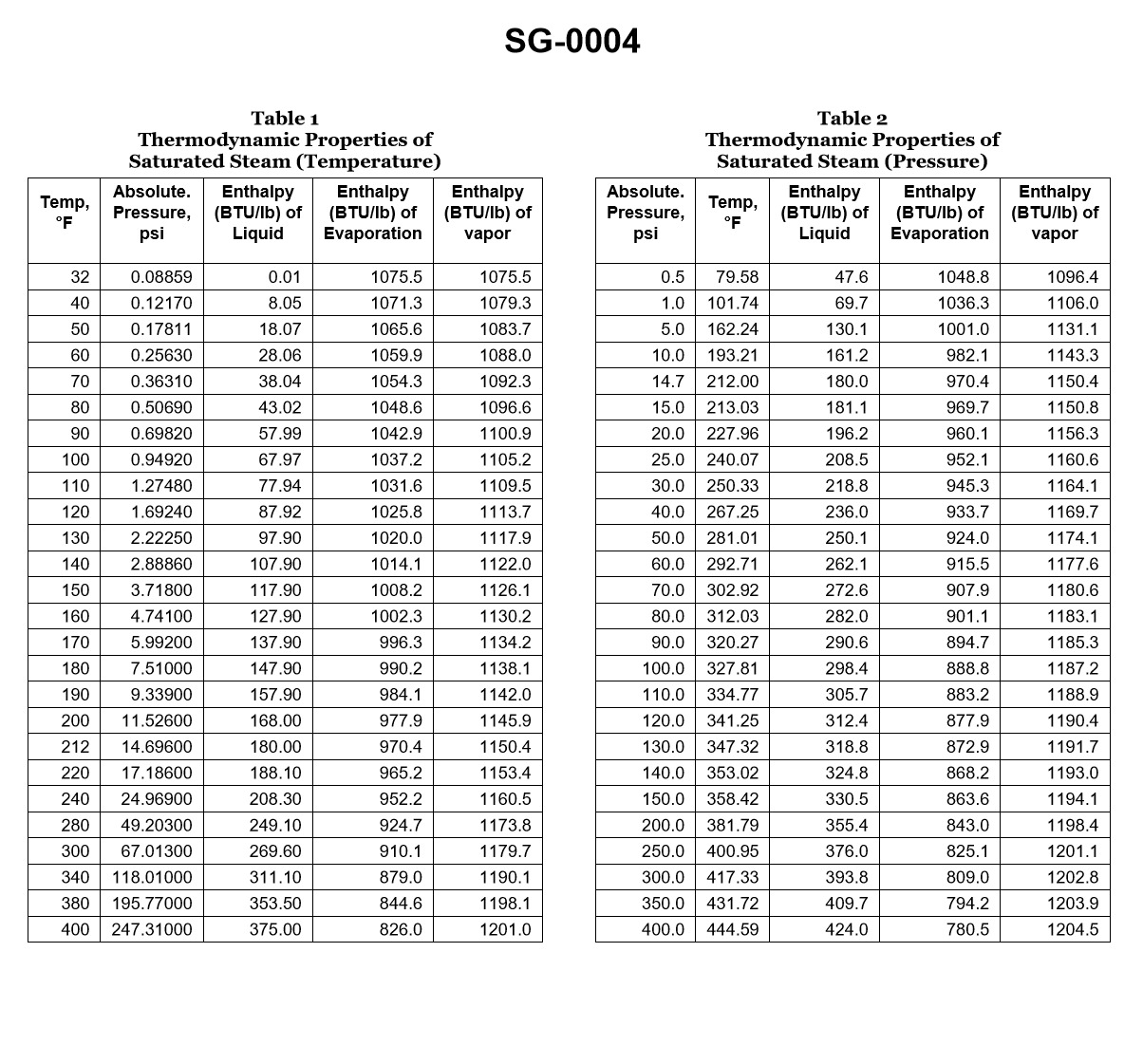
70% to passIf a boiler generates saturated steam at 125.3 psig, how much heat is required to change the water into steam if the feed water temperature is 240°F? Illustration SG-0004
A30.5 Btu/lb
B116.5 Btu/lb
C582.7 Btu/lb
D983.4 Btu/lb
AI Explanation
The correct answer is D) 983.4 Btu/lb. The amount of heat required to change water into saturated steam at a given pressure is known as the latent heat of vaporization. At a pressure of 125.3 psig, the latent heat of vaporization for water is 983.4 Btu/lb. This value can be found in steam tables or other reference materials commonly used for marine engineering calculations. The other options are incorrect because they do not represent the correct latent heat of vaporization for the given pressure. Option A (30.5 Btu/lb) is too low, Option B (116.5 Btu/lb) is also too low, and Option C (582.7 Btu/lb) is significantly lower than the actual value.
Related Questions
Q192:Waterside abrasion of boiler tubes can be caused by _______________. Q22:Boiler refractory previously baked out and fired is most sensitive to __________... Q271:Which of the following actions should be carried out if the boiler water level i... Q260:While underway the engineer on watch reports the vacuum in the main condenser ha... Q194:Boiler tube failures can result from _______________.
Ready to test your knowledge?
Take a Steam Plants - 1st Asst/Chief Practice ExamOfficial Resources