Question 135General Subjects - Assistant Engineer
70% to passWhich of the following statements describes what will occur to the volume of water vapor as it is exposed to the lower temperatures existing in the device labeled "24" shown in the illustration? Illustration MO-0111
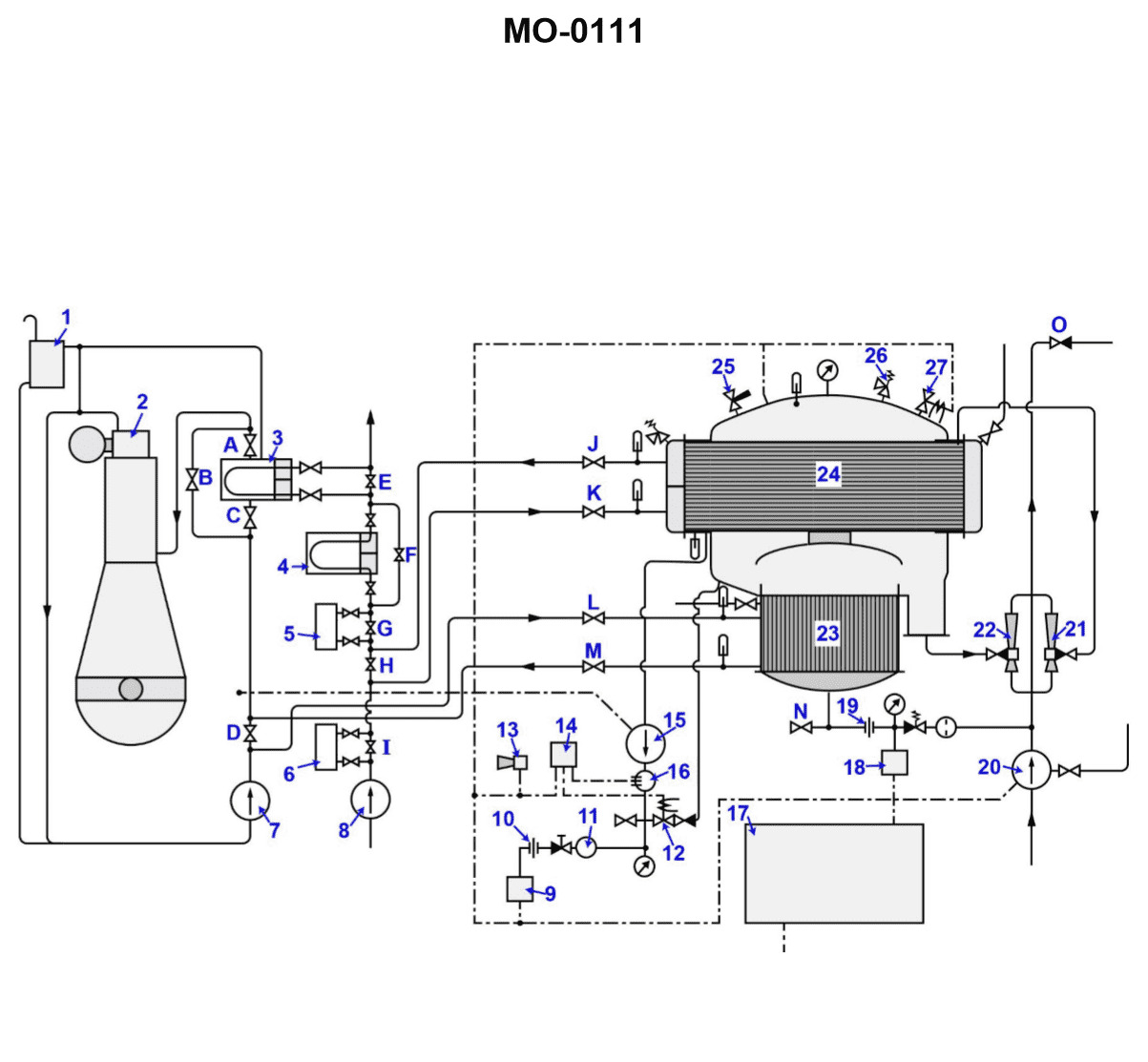
The correct answer is C) The volume is greatly reduced, contributing to condensation within the condenser. As water vapor is exposed to the lower temperatures in the device labeled "24" (which appears to be a condenser), the vapor will condense, causing the overall volume of the water vapor to be greatly reduced. This reduction in volume is a direct result of the condensation process, where the water vapor changes phase from a gas to a liquid, resulting in a much smaller volume compared to the original vapor state. The other answer options are incorrect because: A) The latent heat of condensation is actually released, not removed, during the condensation process. B) The volume decreases, not increases, as the water vapor condenses on the tube surfaces. D) The valve labeled "J" is not a relevant factor in the volume reduction due to condensation within the condenser.
Ready to test your knowledge?
Take a General Subjects - Assistant Engineer Practice Exam