Question 96Electricity & Electronics - Assistant Engineer
70% to passAs shown in the cutaway view of the lead-acid battery in figure "A" of the illustration, if one-half of the battery's cells are revealed by the cutaway section (with the other half remaining hidden from view), what is the nominal output voltage of the battery? Illustration EL-0031
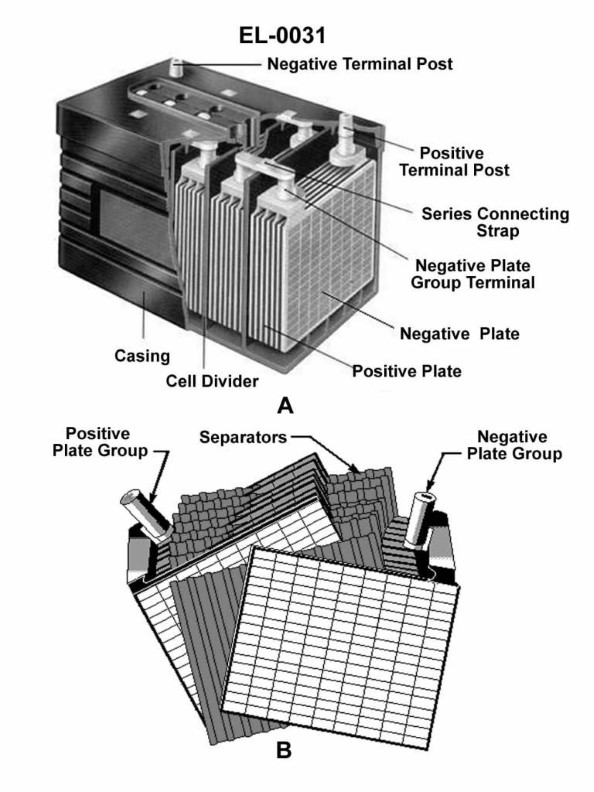
A6 volts
B7.5 volts
C12 volts
D18 volts
AI Explanation
The correct answer is C) 12 volts. The nominal output voltage of a lead-acid battery is determined by the number of cells in the battery, with each cell producing approximately 2 volts. Since the illustration shows that one-half of the battery's cells are revealed, and a lead-acid battery typically has 6 cells, the nominal output voltage of the battery would be 6 cells x 2 volts per cell = 12 volts. The other answer choices are incorrect because 6 volts would represent a battery with only 3 cells, 7.5 volts would not be a standard lead-acid battery voltage, and 18 volts would be too high for a typical lead-acid battery.
Related Questions
Q395:In the illustration of the one-line distribution system diagram, if the ship's s...
Q62:As shown in the illustration, the change-over switch is what type of device? Ill...
Q550:When testing a capacitor with a digital multimeter set up as an ohmmeter, what w...
Q411:As shown in the illustration, which brush holder is of the reaction type? Illust...
Q140:As shown in the illustration, the wet cell storage batteries are connected in wh...
Ready to test your knowledge?
Take a Electricity & Electronics - Assistant Engineer Practice ExamOfficial Resources
Pass Your Coast Guard Licensing Exams!
Study offline, track your progress, and simulate real exams with the Coast Guard Exams app