Question 12 1AE01 - First Assistant Engineer
If valve "H" shown in the illustration is opened wide while the distiller is in operation, __________. Illustration MO-0111
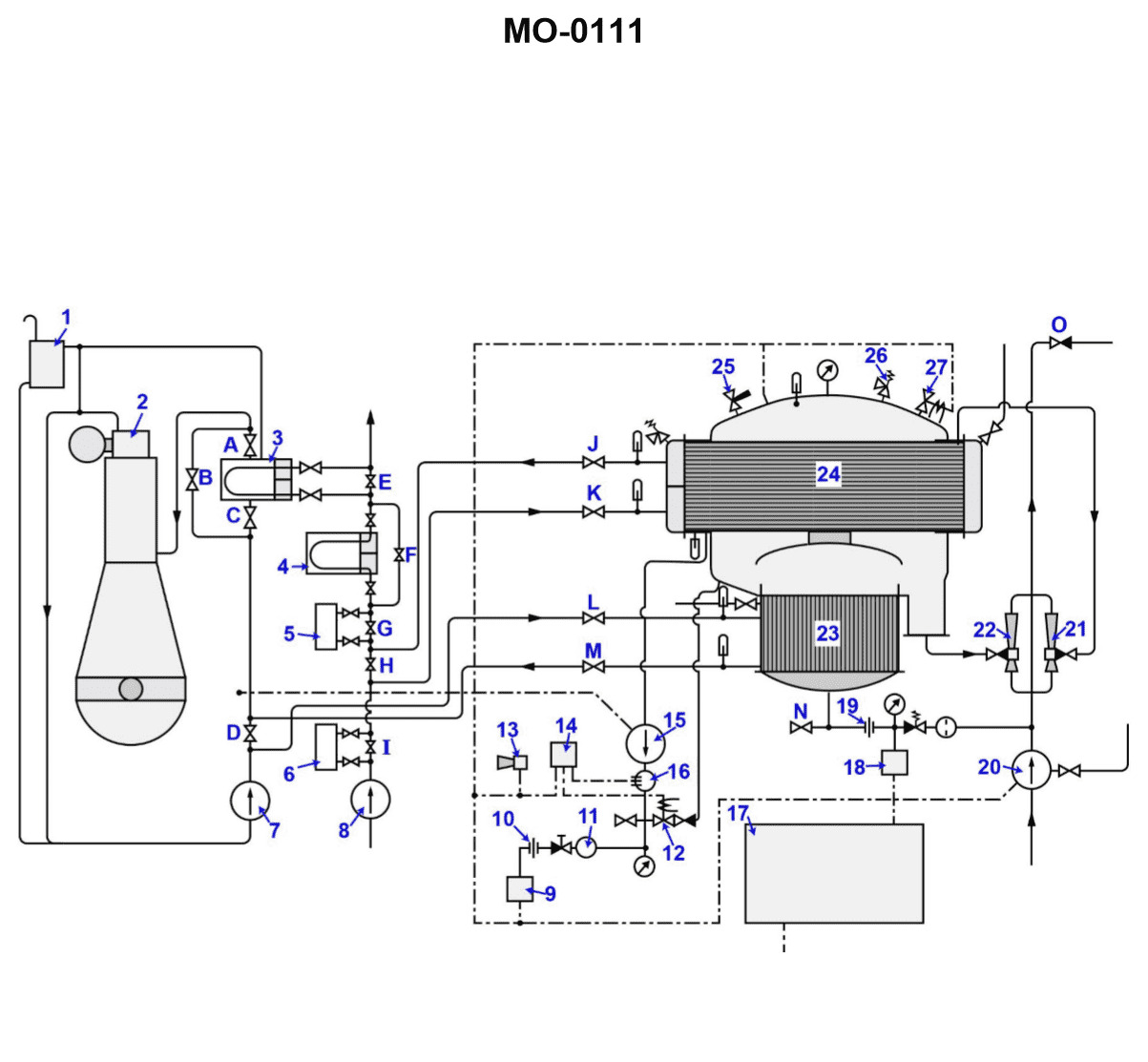
The Correct Answer is C ### Explanation for Option C (Correct) Option C states that the absolute pressure of the unit will increase with an associated increase in shell temperature. This is correct because valve "H" is typically the air ejector bypass valve (or steam line bypass valve, depending on the specific MO-0111 diagram, but functionally, opening it wide compromises the vacuum system). The primary function of the air ejector (or vacuum pump system) is to remove non-condensable gases (mainly air) from the evaporator shell, which maintains a high vacuum (very low absolute pressure). 1. **Opening Valve H Wide:** If valve "H" (the air ejector bypass or a critical steam/vacuum isolation valve that controls the vacuum achieved by the ejector) is opened wide, it typically allows high-pressure steam (if it's a bypass) to leak into the low-pressure side, or it introduces a significant leak path, or it allows the vacuum system to work inefficiently. In the context of shell-and-tube distillers (like standard flash or low-pressure evaporators), opening the ejector bypass wide would reduce the effective vacuum created by the ejector, often by starving the nozzles or creating back pressure. 2. **Pressure Increase:** When the effectiveness of the vacuum system is compromised, the concentration of non-condensable gases and water vapor pressure increases inside the evaporator shell. This results in a significant **increase in the absolute pressure** of the unit (the vacuum worsens). 3. **Temperature Increase:** According to the principles of saturated steam tables, there is a direct correlation between saturation pressure and saturation temperature. Since the water being boiled must match the saturation temperature corresponding to the pressure inside the shell, an **increase in absolute pressure** must lead to an **increase in the associated shell temperature** (the boiling point of the water). ### Explanation of Incorrect Options **A) the absolute pressure of the unit will not be affected, but the rate of condensation will be decreased:** This is incorrect. As explained above, opening the vacuum bypass or starving the air ejector drastically affects the vacuum, causing the absolute pressure to increase. The decrease in condensation rate is a consequence of the pressure and temperature changes, but the core premise that pressure is unaffected is wrong. **B) the absolute pressure of the unit will increase due to the increased effect of the air ejector:** This is incorrect. While the absolute pressure *will* increase, it is due to the *decreased* (or compromised) effect of the air ejector, not an increased effect. An efficient air ejector decreases pressure; compromising it increases pressure. **D) the absolute pressure of the unit will increase with an associated decrease in shell temperature:** This is incorrect. While the absolute pressure will increase, the shell temperature (boiling point) is directly proportional to the absolute pressure in a saturated system. Therefore, an increase in pressure is always associated with an increase in the shell temperature.
Pass Your Coast Guard Licensing Exams!
Study offline, track your progress, and simulate real exams with the Coast Guard Exams app